Chemical Reaction Types( Decomposition)
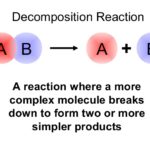
A chemical decomposition reaction or analysis reaction is one of the most common types of chemical reactions. In a decomposition reaction a compound is broken into smaller chemical species.
AB → A + B
The electrolysis of water into oxygen and hydrogen gas is an example of a decomposition reaction:
2 H2O → 2 H2 + O2
Another example is the decomposition of potassium chloride into potassium and chlorine gas.
2 KCl(s) → 2 K(s) + Cl2(g)
Types of Decomposition Reactions
Decomposition by Sunlight
Decomposition due to Heat
Decomposition due to Acids